Lewis acid-promoted site-selective cyanation of phenols†
Abstract
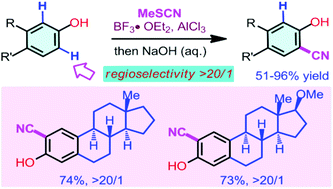
An efficient Lewis acid-promoted site-selective electrophilic cyanation of 3-substituted and 3,4-disubstituted phenols has been developed. The cyanation reactions using MeSCN as the cyanating reagent proceeded efficiently to afford a wide range of 2-hydroxybenzonitriles with high efficiency and excellent regioselectivity. This protocol could provide a practical method for the synthesis and modification of biologically active molecules.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...