Dienaminodioate based multicomponent reactions with post-benzylic oxidative transformations mediated by DDQ†
Abstract
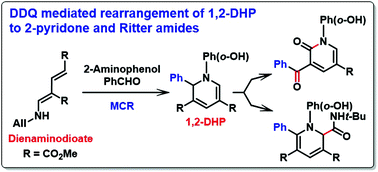
Multicomponent reactions (MCRs) using dienaminodioate with post-benzylic oxidative transformation mediated by DDQ that afforded a diverse array of products are described. An unprecedented rearrangement of 1,2-dihydropyridines (1,2-DHPs), 3CR products, to 2-pyridones in good yields with a broad substrate scope by DDQ-mediated benzylic oxidation via a pyridinium intermediate is reported. Treatment of the pyridinium intermediate with tert-butyl isocyanide afforded isomerized 1,2-DHPs, analogous to Ritter amides. Further diversification using 3CR products bearing a benzylic group, predictably, promoted the synthesis of 2-pyridone with a benzylideneamine group and a benzo[d]oxazole appended biaryl group by DDQ. A formal 1,6-reduction product from 2-pyridone in the presence of NaBH4 is also observed.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...