Recent development in the synthesis of C-glycosides involving glycosyl radicals
Abstract
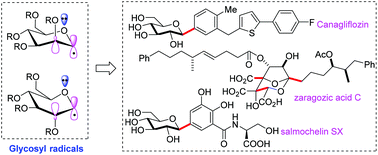
C-Glycosylation involving glycosyl radical intermediates is a particularly effective approach to access C-glycosides, which are core units of a great number of natural products, bioactive compounds and marketed drugs. In this review, we summarize the progress of glycosyl radical-based C-glycoside synthesis between 1999–2020, focusing on the stereoselectivity and recently developed methodologies such as α-alkoxyacyl telluride-related, photo-mediated and transition-metal catalysed reactions. Metal-mediated reductive cross coupling is also covered due to its close relationship with the latter approaches. To introduce several strategies for achieving uncommon β-stereoselective C-glycosylation, we also briefly described organotin-based methods.
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...