Light-induced one-pot synthesis of pyrimidine derivatives from vinyl azides†
Abstract
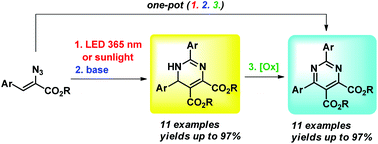
A one-pot procedure for the synthesis of tetrasubstituted dihydropyrimidine and pyrimidine derivatives from α-azidocinnamates was developed. The synthesis is based on the finding that the outcome of LED photolysis of α-azidocinnamates depends on the light wavelength employed. Blue light (455 nm) leads to the formation of 2H-azirines only, but violet light (395 nm), UV-A light (365 nm), or sunlight result in the transformation of the in situ formed 2H-azirines to 1,3-diazabicyclo[3.1.0]hex-3-enes. Under basic catalysis (DBU), the latter were isomerized to 1,6-dihydropyrimidines which were oxidized to pyrimidines using DDQ. A successful use of Cs2CO3 as a base and air as an oxidant was also demonstrated.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...