Synthesis of B-ring-fluorinated (−)-epicatechin gallate derivatives†
Abstract
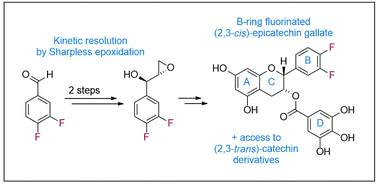
The synthesis of enantiomerically pure B-ring fluorinated catechin derivatives is presented. In a convergent approach the chromane was obtained by reaction of a lithiated fluoro-resorcine with an optically active epoxide. The latter was prepared from 3,4-difluorobenzaldehyde by reaction with vinylmagnesium bromide followed by Sharpless epoxidation. The protocol provides access to both fluorinated catechin as well as epicatechin derivatives.
- This article is part of the themed collection: Total synthesis in OBC


 Please wait while we load your content...
Please wait while we load your content...