Abstract
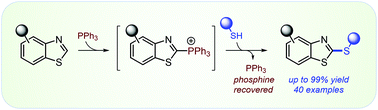
Thiazoles and benzothiazoles undergo regioselective C2–H chalcogenation via the sequence of thiazole C2-functionalization with phosphines to produce phosphonium salts which in turn react with S- and Se-centered nucleophiles to give products of C2–H chalcogenation and allow for recovery of the starting phosphine. The atom economical sequence proceeds under mild conditions and features broad scope for both the nucleophiles (electron-rich, electron-poor, sterically hindered thiols) and the various substituted benzothiazoles. The access to the substituted medicinally relevant C2-thio benzothiazoles also enables stereoselectivity improvements in the modified Julia olefinations.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...