Synthesis of unsymmetrical urea derivatives via one-pot sequential three-component reactions of cyclic 2-diazo-1,3-diketones, carbodiimides, and 1,2-dihaloethanes†
Abstract
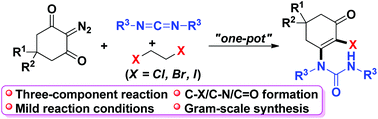
A novel and efficient multicomponent reaction of cyclic 2-diazo-1,3-diketones, carbodiimides, and 1,2-dihaloethanes has been developed, and it leads to unsymmetrical urea derivatives with good yields in a single operation. This transformation involves the cascade formation of C–X (X = Cl, Br, I), C–N and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds through halogenation, nucleophilic addition, ring-opening, and enol–ketone tautomerization processes. This protocol is step- and atom-economical; 1,2-dihaloethane was used as an easily available halogenated reagent, and it is amenable to different functional groups.
O bonds through halogenation, nucleophilic addition, ring-opening, and enol–ketone tautomerization processes. This protocol is step- and atom-economical; 1,2-dihaloethane was used as an easily available halogenated reagent, and it is amenable to different functional groups.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...