Comparison of disaccharide donors for heparan sulfate synthesis: uronic acids vs. their pyranose equivalents†
Abstract
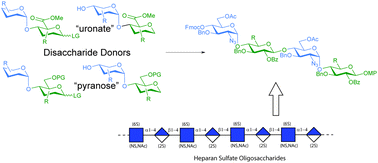
Late oxidation of hexose based building blocks or the use of uronic acid containing building blocks are two complementary strategies in the synthesis of glycosaminoglycans, the latter simplifiying the later stages of the process. Here we report the synthesis and evaluation of various disaccharide donors—uronic acids and their pyranose equivalents—for the synthesis of heparan sulfate, using an established protective group strategy. Hexose based “imidate” type donors perform well in the studied glycosylations, while their corresponding uronate esters fall short; a uronate ester thioglycoside performs equal to, if not better than, a hexose thioglycoside equivalent.
- This article is part of the themed collections: Chemical Biology in OBC and Glycosylation: New methodologies and applications


 Please wait while we load your content...
Please wait while we load your content...