One-pot synthesis of pyrimidine linked naphthoquinone-fused pyrroles by iodine-mediated multicomponent reactions†
Abstract
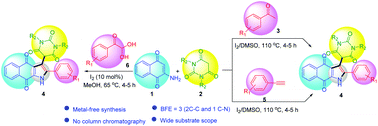
Herein we report iodine-mediated multicomponent reactions for the synthesis of naphthoquinone-fused pyrroles tethered with a pyrimidine moiety. The reaction of aryl methyl ketones (3) or terminal aryl alkynes (5) in the presence of molecular iodine in DMSO medium followed by sequential addition of barbituric acids (2) and 2-amino-1,4-naphthoquinone (1) provides the corresponding three-component hybrid molecules 4 having naphthoquinone-fused pyrroles tethered with a barbituric acid moiety. This three-component reaction proceeds via metal-free C–H oxidation followed by multi-component cyclization forming three new bonds (2 C–C, 1 C–N) in one pot. Alternatively, the same molecules can also be prepared from the reaction of arylglyoxals, 2-aminonaphthoquinone, and barbituric acids in the presence of a catalytic amount of iodine in methanol medium under reflux conditions. The salient features of these methodologies are: one-pot metal-free method, good yields, wide substrate scope, easy purification of products, and the presence of medicinally important naphthoquinone, pyrrole and pyrimidine moieties in the products.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...