Divergent syntheses of okaramines C, J, L, and S–U†
Abstract
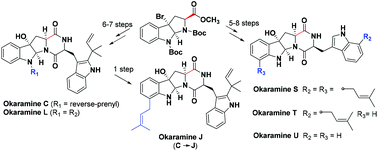
The total synthesis of six novel okaramines (C, J, L, and S–U) was accomplished with a precise synthesis scheme involving a few steps and a practical yield of 6.7%–23% was obtained. The significance of this study includes the design of a successful and convenient synthesis method for preparation of 3a-hydroxy-pyrrolo[2,3-b]-indole and C-7 prenylated L-tryptophan derivatives using a nucleophilic attack of cyclopropylazetoindoline and an aza-Claisen rearrangement of N-reverse-prenyl tryptophan, respectively.
- This article is part of the themed collection: Total synthesis in OBC


 Please wait while we load your content...
Please wait while we load your content...