Organocatalytic asymmetric addition of thioglycolates to o-quinone methides: a route to 5-substituted-5H-benzoxathiepine-2(3H)-ones†
Abstract
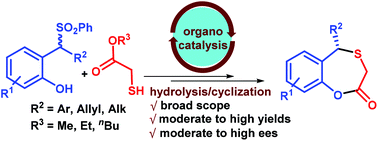
Herein we have developed the first enantioselective synthesis of 5-substituted-5H-benzoxathiepine-2(3H)-ones. 2-Sulfonylmethyl phenols and thioglycolates were employed as reaction partners in this method. The desired thia Michael products were obtained via bifunctional squaramide catalyzed conjugate addition reaction to in situ generated o-quinone methides and then basic hydrolysis followed by cyclization led to the formation of 5-substituted-5H-benzoxathiepine-2(3H)-ones. Broad scope and moderate to high enantioselectivities were observed for both products.
- This article is part of the themed collections: Synthetic methodology in OBC and Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...