Enantioselective synthesis of indanone spiro-isochromanone derivatives via a dinuclear zinc-catalyzed Michael/transesterification tandem reaction†
Abstract
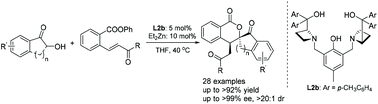
An enantioselective Michael/transesterification tandem reaction of α-hydroxy indanones with ortho-ester chalcones was realized using dinuclear zinc catalysts. A series of enantiomerically pure spiro[indanone-2,3′-isochromane-1-one] derivatives were obtained in good yields with excellent stereoselectivities (up to >20 : 1 dr, up to >99% ee). This protocol could be conducted on a gram scale without affecting its stereoselectivities. In addition, the absolute stereochemistry of the products was determined by X-ray crystallographic analysis of 3ac, and a positive nonlinear effect was observed. Finally, a possible catalytic cycle was proposed to explain the origin of the enantioselectivity.
- This article is part of the themed collections: Synthetic methodology in OBC and Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...