The ruthenium(ii)-catalyzed C–H olefination of indoles with alkynes: the facile construction of tetrasubstituted alkenes under aqueous conditions†
Abstract
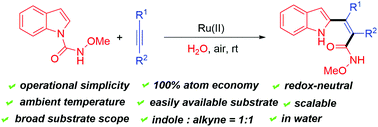
An environmentally-friendly and facile protocol for the construction of tetrasubstituted alkenes has been established with Ru(II)-catalyzed C–H bond functionalizations under mild conditions. The method features the usage of readily available substrates, without external oxidants and additives, 100% atom economy, and excellent regioselectivity, thus enhancing the practicability of this protocol. Moreover, this transformation proceeded smoothly under aqueous conditions and could be extended to the gram scale. N-Methoxyamide, as a directing group (DG), played a vital role in the transformation.


 Please wait while we load your content...
Please wait while we load your content...