Synthesis, structures and photophysical properties of hexacoordinated organosilicon compounds with 2-(2-pyridyl)phenyl groups†
Abstract
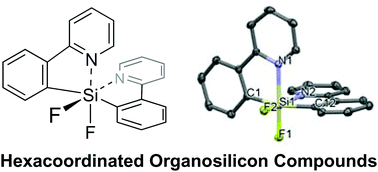
We synthesised novel hexacoordinated organosilicon compounds with two 2-(2-pyridyl)phenyl groups. Single-crystal X-ray structure analyses indicated that Lewis acid–base interactions exist between the silicon atom and two nitrogen atoms of the pyridine rings, and that hexacoordinated organosilicon compounds have slightly distorted octahedral structures in the solid state. The hexacoordinated organosilicon compounds are stable in air, water, heat, acids, and bases. The fluorescent quantum yield increased dramatically and a significant red-shift of the maximum fluorescence wavelength was observed with the introduction of amino groups on the 2-(2-pyridyl)phenyl aromatic rings. The fluorescence colours of a hexacoordinated organosilicon compound with two amino groups can be switched by protonation and deprotonation (neutralisation) of the amino groups.


 Please wait while we load your content...
Please wait while we load your content...