Efficient diversification of GM3 gangliosides via late-stage sialylation and dynamic glycan structural studies with 19F solid-state NMR†
Abstract
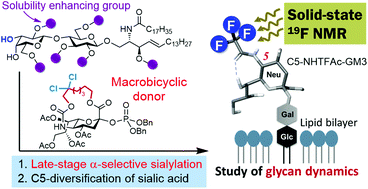
Sialic acid-containing glycoconjugates are involved in important biological processes such as immune response, cancer metastasis, and viral infection. However, their chemical syntheses have been challenging, mainly due to the difficulties in the α-sialylation of oligosaccharides. Very recently, we established a completely stereoselective sialidation method using a macrobicyclic sialyl donor. Herein, we describe a rational and efficient synthesis of sialoglycolipids via direct sialylation of a glycolipid at a late-stage, based on our novel sialidation method. The synthetic method enabled the development of GM3 ganglioside analogs with various C5-modifications of the sialosyl moiety. Furthermore, the synthesized analog was subjected to solid-state 19F NMR analysis on the model membranes and it revealed the influence of cholesterol on glycan dynamics.
- This article is part of the themed collections: Glycosylation: New methodologies and applications and Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...