Non-biaryl atropisomerism at the C–B bond in sterically hindered aminoarylboranes†
Abstract
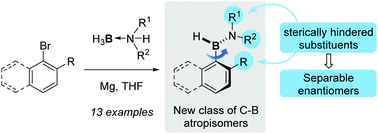
Sterically hindered aminoarylboranes featuring atropisomerism about the C–B bond were prepared by addition of organomagnesium species onto readily accessible dialkylamine–borane complexes. Some of these aminoarylboranes, isosteres of vinyl styrene derivatives, were resolved by HPLC on the chiral stationary phase. They are the first examples of a non-biaryl type system which display slow rotation about a C–B bond.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...