Organocatalytic asymmetric cascade 1,6-addition/hemiketalization/retro-Henry reaction of ortho-hydroxyphenyl-substituted p-QMs with α-nitroketones†
Abstract
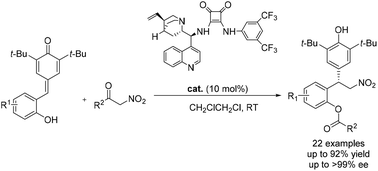
A novel organocatalytic asymmetric cascade 1,6-addition/hemiketalization/retro-Henry reaction of ortho-hydroxyphenyl-substituted p-QMs with α-nitroketones is described. A variety of novel chiral 2-(1-(4-hydroxyphenyl)ethyl)phenols are constructed for the first time with high yields (up to 92%) and excellent enantioselectivities (up to >99% ee) under mild reaction conditions. A gram-scale experiment of this process is also presented.
- This article is part of the themed collection: Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...