Regioselective catalytic asymmetric N-alkylation of isoxazol-5-ones with para-quinone methides†
Abstract
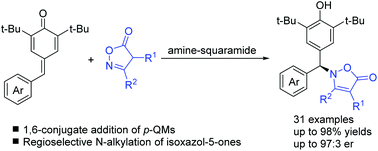
A highly regioselective and enantioselective N-alkylation of isoxazol-5-ones with para-quinone methides promoted by bi-functional squaramide catalysts was developed. This unexpected asymmetric N-addition of isoxazolinones afforded a series of enantioenriched N-diarylmethane substituted isoxazolinones with high yields and enantioselectivities (up to 97 : 3 er). This reaction not only provides a useful approach for intermolecular chiral C–N bond formation but also demonstrates the immense potential of isoxazol-5-ones as N-nucleophiles in catalytic asymmetric reactions.
- This article is part of the themed collections: Synthetic methodology in OBC and Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...