Stereoselective preparation of P,axial-stereogenic allenyl bisphosphine oxides via chirality-transfer†
Abstract
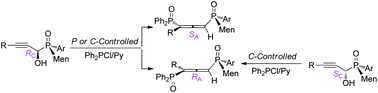
P,C-Stereogenic propargyl alcohols RC-3/SC-3′ were prepared by the addition of (L)-menthyl-derived SPOs to propynals, which were converted to P,axial-stereogenic allenyl bisphosphine oxides. The chirality transfer was controlled by α-carbon via syn [2,3]-sigmatropic rearrangement. For SC-3′ linking weak WDG on the alkynyl moiety, the chirality on the axis depended on stereogenic phosphorus.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...