Access to chiral α-substituted-β-hydroxy arylphosphonates enabled by biocatalytic dynamic reductive kinetic resolution†
Abstract
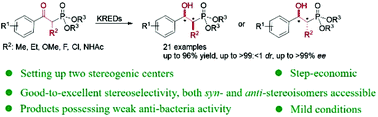
Ketoreductase (KRED)-catalyzed dynamic reductive kinetic resolution (DYRKR) of α-substituted-β-keto arylphosphonates was developed as a generic and stereoselective approach to synthesize chiral α-substituted-β-hydroxy arylphosphonates, with moderate-to-excellent isolated yield (up to 96%), good-to-excellent diastereoselectivity (up to >99 : <1 dr), and excellent enantioselectivity (up to >99% ee) being achieved.
- This article is part of the themed collections: Synthetic methodology in OBC and Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...