1,2-cis-Selective glucosylation enabled by halogenated benzyl protecting groups†
Abstract
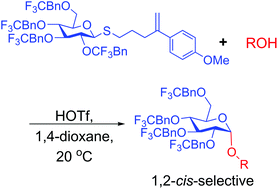
We report on our initial results from a systematic effort to implement electron-withdrawing protecting groups and Lewis basic solvents/additives as an approach to 1,2-cis(α)-selective O-glucosylation. 1,2-cis-Selective O-glucosylations are reported with thioglucosides and glucosyl trichloroacetimidates and a range of acceptors. A correlation between electron-withdrawing effects and 1,2-cis selectivity has been established. This phenomenon may prove to be broadly applicable in the area of chemical O-glycosylation.
- This article is part of the themed collection: Glycosylation: New methodologies and applications


 Please wait while we load your content...
Please wait while we load your content...