Diversity oriented multi-component reaction (DOS–MCR) approach to access natural product analogues: regio- and chemo-selective synthesis of polyheterocyclic scaffolds via one-pot cascade reactions†
Abstract
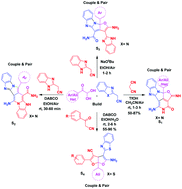
Skeletally diverse and complex aza-cyclopenta(cd)diindene, pyrrolo(3,4-d)pyridine-13-carboxamide, and furo-pyrrolo(1,2-a)imidazole-4-carboxamide fused polyheterocyclic hybrid scaffolds and a furo(2,3-b)furan core have been accessed via one-pot three-component reaction by exploiting the build/couple/pair strategy of diversity oriented synthesis (DOS). This protocol is metal free, has a good substrate scope and affords products with good to excellent yields and regio- and chemo-selectivity. The heterocyclic skeletons obtained in this study mimic natural products such as eupolauramine, gracilamine and presilphiperfolanol.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...