Spontaneous oxidative cyclisations of 1,3-dihydroxy-4-dimethylallylnaphthalene to tricyclic derivatives†
Abstract
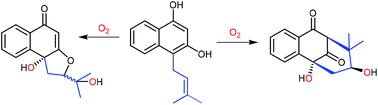
The attachment of a dimethylallyl moiety to C4 of 1,3-dihydroxynaphthalene led to spontaneous oxidative cyclisations, resulting in the formation of two tetrahydrobenzofuran and one bicyclo[3.3.1]nonane derivatives. Incubation under an 18O-rich atmosphere proved that both the incorporated oxygen atoms originated from O2. A radical-involved mechanism is proposed for these cyclisations.
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...