Synthesis of alkynes from non-alkyne sources
Abstract
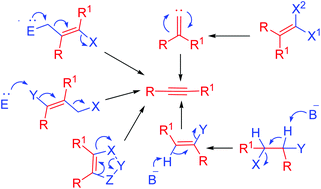
Alkynes are a very important class of compounds and used as precursors for the assembly of a variety of carbocycles and heterocycles. Alkynes are classified into two classes: terminal alkynes and internal alkynes. Terminal alkynes were used as one of the precursors for the synthesis of internal alkynes. Besides, various elimination reactions were conducted to obtain alkynes. Various applications of alkynes are known but no compiled review is reported so far regarding their synthesis. Therefore, we have compiled the literature available for the synthesis of various functionalized alkynes from non-alkyne sources in this review. We have not discussed the synthesis of internal alkynes from terminal alkynes. Based on the available literature, the synthesis of alkynes can be classified into three major types of reactions: (a) synthesis of alkynes through α,β-elimination, (b) synthesis of alkynes through carbene rearrangement and (c) synthesis of alkynes via miscellaneous approaches. In this review, we have focused on the different methods available for these transformations including their scope, limitations and recent developments. We have also discussed the mechanism involved during the reaction.


 Please wait while we load your content...
Please wait while we load your content...