1,2-Addition to trifluoromethylated β-enamino diketones: regioselective synthesis of trifluoromethyl-containing azomethine pyrazoles and isoxazoles†
Abstract
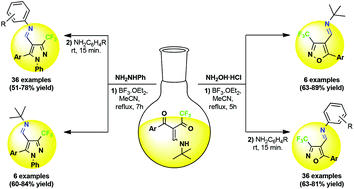
A simple and efficient methodology for highly regioselective synthesis of azomethine pyrazoles and isoxazoles containing a trifluoromethyl group is reported. The cyclocondensation of trifluoromethylated β-enamino diketones (TBED) with phenylhydrazine and hydroxylamine hydrochloride, in the presence of BF3, provided 5-aryl-4-[(tert-butyl)iminomethyl]-3-trifluoromethylazoles by aza-Michael-type 1,2-addition. The scope of the reaction was expanded by transimination with arylamines in a one-pot method starting from TBED. Thus, 83 novel 4-[(alkyl/aryl)iminomethyl]-3-trifluoromethylazoles were obtained with high regioselectivity and in yields of 51 to 89%.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...