Metal-free and regiospecific synthesis of 3-arylindoles†
Abstract
A convenient, metal-free, and organic acid–base promoted synthetic method to prepare 3-arylindoles from 3-aryloxirane-2-carbonitriles and arylhydrazine hydrochlorides has been developed. In the reaction, the organic acid catalyzes a tandem nucleophilic ring-opening reaction of aryloxiranecarbonitriles and arylhydrazine hydrochlorides and Fischer indolization. The organic base triethylamine plays a crucial role in the final elimination step in the Fischer indole synthesis, affording 3-arylindoles regiospecifically. The reaction features advantages of microwave acceleration, non-metal participation, short reaction time, organic acid–base co-catalysis, and broad substrate scope.
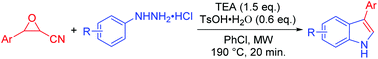
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...