Acid–base-sensitive allylic oxidation of 2-allylbenzoic acids to form phthalides†
Abstract
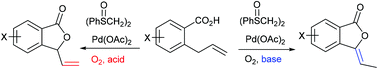
Allylic oxidation of 2-allylbenzoic acids to phthalides, instead of Wacker-type isocoumarins, was achieved with 1,2-bis(phenylsulfinyl)ethane palladium(II) acetate (White catalyst) and oxygen in DMSO. The selective formation of 3-ethylidenephthalides or 3-vinylphthalides was controlled by the addition of acids or bases, and the reaction conditions were applied to substituted 2-allylbenzoic acids to generate corresponding phthalides selectively. Mechanistic studies, including the corresponding reaction of (E)-2-(1-propenyl)benzoic acid to 3-methylisocoumarin, isomerization reaction of 3-vinylphthalide to 3-ethylidenephthalide, and the kinetic isotope effect using 2-(1,1-d2-allyl)benzoic acid, revealed the competition between Wacker-type oxidation and allylic C–H cleavage, which is the key step to generating phthalides. A natural product, 3-ethyl-6-hydroxyphthalide, was prepared by this method.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...