Photochemical rearrangement of diarylethenes: synthesis of functionalized phenanthrenes†
Abstract
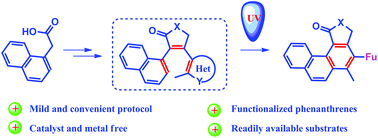
A novel protocol for the synthesis of functionalized phenanthrenes through photocyclization of diarylethenes (DAE) under UV irradiation is proposed. The reaction proceeds through 6π-electrocyclization with the formation of a cyclic (closed) intermediate that undergoes a rearrangement affording unsymmetrical phenanthrenes in good yields. However, in contrast to benzene derivatives, the photocyclization of naphthalene diarylethenes proceeds more slowly, which is confirmed by DFT calculations. The transformation was performed on a 1 mmol scale. The scalability showed that the diarylethenes bearing oxazole, thiazole, pyrazole and imidazole as aryl moieties are more prone to photorearrangement and can be used in preparative organic synthesis.


 Please wait while we load your content...
Please wait while we load your content...