Controlling the selectivity of an intramolecular Friedel–Crafts alkylation with alkenes using selenium under mild conditions†
Abstract
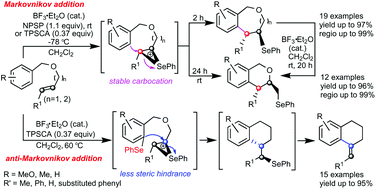
An efficiently divergent intramolecular Friedel–Crafts alkylation by unactivated alkenes with seleniranium ion-controlled Markovnikov/anti-Markovnikov specificities under mild conditions has been investigated. 2-Benzoxepin, isochroman, and isochromene can be produced in one-pot procedures from the same substrate in high yields and with high regio- and stereospecificity. The products are challenging to access via 7-endo-trig carbocyclizations and by 7-endo-trig carbocyclization/rearrangement/6-exo-trig oxycyclization and 6-exo-trig carbocyclization/deselenenylation reaction sequences, respectively. Mechanistic experiments indicated that in addition to the stereospecific anti-addition processes of the cyclization reactions, the formation of a stable carbocation after ring opening of the seleniranium ion leads to an NPSP-mediated 7-endo-trig carbocyclization; the steric hindrance of the seleniranium intermediate controls the regioselectivity when using TPSCA at 60 °C, which promotes 6-exo-trig carbocyclization. Two distinct catalytic cycles were proposed, and the structures of transition states and products were identified by ab initio calculations and X-ray analyses.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...