Synthesis of β-lactams via diastereoselective, intramolecular Kinugasa reactions†
Abstract
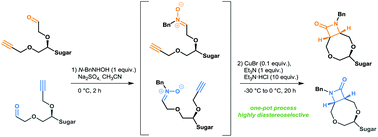
Intramolecular Kinugasa reactions on in situ generated carbohydrate-derived alkynylnitrones are described. The effects of the length of chains, their mutual configuration, influence of experimental conditions on product distribution and feasibility of the β-lactam ring construction were studied. Intramolecular reactions proceed with high stereoselectivity to provide in each case one product only. The cycloadducts from tartaric acid were converted into the corresponding non-racemic 4-acetoxy azetidinones in good yields.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...