Base-promoted Lewis acid catalyzed synthesis of quinazoline derivatives†
Abstract
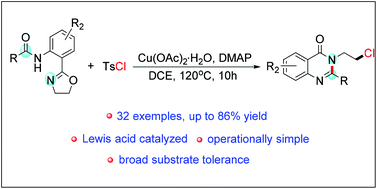
A one-pot protocol has been developed for the synthesis of quinazolinones from amide-oxazolines with TsCl via a cyclic 1,3-azaoxonium intermediate and 6π electron cyclization in the presence of a Lewis acid and base. The process is operationally simple and has a broad substrate scope. This method provides a unique strategy for the construction of quinazolinones.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...