Direct synthesis of cyclic lipopeptides using intramolecular native chemical ligation and thiol–ene CLipPA chemistry†
Abstract
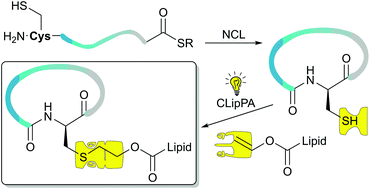
Naturally occurring cyclic lipopeptides exhibit a diverse range of biological activities and possess several favourable properties. Chemically synthesising and modifying these natural compounds can alter their biological and physical properties. Cyclic lipopeptides are often difficult to synthesise, especially when the lipid moiety is directly attached to the cyclic scaffold. The construction of a series of cyclic lipopeptide analogues of the antifungal peptide iturin A is reported herein. The synthesis of the parent peptide macrocycle was achieved using native chemical ligation (NCL), whereupon the regenerated free thiol was used to attach a lipid moiety using Cysteine Lipidation on a Peptide or Amino acid (CLipPA) technology.


 Please wait while we load your content...
Please wait while we load your content...