Electrochemically enabled functionalization of indoles or anilines for the synthesis of hexafluoroisopropoxy indole and aniline derivatives†
Abstract
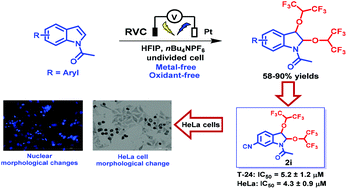
An environmentally benign electrochemically enabled site-selective functionalization of indole or aniline derivatives with hexafluoroisopropanol in the presence of tetrabutyl ammonium hexafluorophosphate as the redox catalyst and electrolyte was demonstrated in this work. Under mild electro-oxidation conditions, a series of hexafluoroisopropoxy indole and aniline derivatives with pharmacological activity were obtained. This conversion does not need transition metals and oxidants, and has good functional group tolerance. The in vitro cytotoxicity of all compounds was evaluated by the MTT assay against four human cancer cell lines. The results revealed that hexafluoroisopropoxy indoles have good antitumor activity and compound 2i increased the intracellular levels of ROS and inhibited apoptosis in HeLa cells.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...