Mutational biosynthesis to generate novel analogs of nosiheptide featuring a fluorinated indolic acid moiety†
Abstract
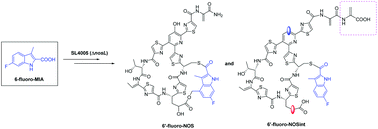
Nosiheptide (NOS) is a member of bicyclic thiopeptides possessing a biologically important indolic acid (IA) moiety appended onto the family-characteristic core system. The IA formation relies primarily on NosL, a radical S-adenosylmethionine (SAM) protein that catalyzes a complex rearrangement of the carbon side chain of L-tryptophan, leading to the generation of 3-methyl-2-indolic acid (MIA). Here, we establish an efficient mutational biosynthesis strategy for the structural expansion of the side-ring system of NOS. The nosL-deficient mutant Streptomyces actuosus SL4005 complemented by chemically feeding 6-fluoro-MIA is capable of accumulating two new products. The target product 6′-fluoro-NOS contains an additional fluorine atom at C6 of the IA moiety, in contrast with an unexpected product 6′-fluoro-NOSint that features an open side ring and a bis-dehydroalanine (Dha) tail. The newly obtained 6′-fluoro-NOS displayed equivalent or slightly reduced activities against the tested drug-resistant pathogens compared with NOS, but dramatically decreased water solubility compared with NOS. Our results indicate that the modification of the IA moiety of NOS not only affects its biological activity but also affects its activity which will be key considerations for further modification.
- This article is part of the themed collections: Chemical Biology in OBC and Methodology development for protein modifications


 Please wait while we load your content...
Please wait while we load your content...