Migratory ability of quinone methide-generating acridine conjugates in DNA†
Abstract
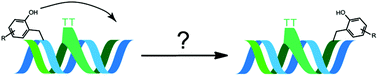
The dynamic nature of nucleic acid alkylation by simple ortho quinone methides (QM) and their conjugates has provided numerous opportunities ranging from sequence selective targeting to bipedal walking in duplex DNA. To enhance the diffusion rate of adduct migration, one of two sites for QM generation was deleted from a bisQM conjugate of acridine to remove the covalent anchor to DNA that persists during QM regeneration. This conversion of a bisfunctional cross-linking agent to a monofunctional alkylating agent allowed adduct diffusion to traverse an extrahelical -TT- bulge that previously acted as a barrier for its bisfunctional analog. An electron rich derivative of the monofunctional acridine conjugate was additionally prepared to accelerate the rates of DNA alkylation and QM regeneration. The resulting stabilization of this QM effectively enhanced the rate of its release from adducts attached at guanine N7 in competition with an alternative and detrimental deglycosylation pathway. Intercalation by the acridine component was not sufficient to hold the transient QM intermediates within duplex DNA and consequently these electrophiles diffused into solution and were subject to quenching by solvent and a model nucleophile, β-mercaptoethanol.
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...