Abstract
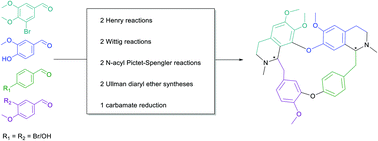
An efficient racemic total synthesis of the bisbenzylisoquinoline alkaloids tetrandrine and isotetrandrine in four different routes is reported herein. Key steps of the synthesis include N-acyl Pictet–Spengler condensations to access the tetrahydroisoquinoline moieties, as well as copper-catalyzed Ullmann couplings for diaryl ether formation. Starting from commercially available building blocks tetrandrine and isotetrandrine are accessed in 12 steps. Depending on the sequence of the four central condensation steps, equimolar mixtures of both diastereomers or predominantly tetrandrine or its diastereomer isotetrandrine are obtained. Through computational analysis we were able to rationalize the differences in the observed diastereomeric specificities.
- This article is part of the themed collection: Total synthesis in OBC


 Please wait while we load your content...
Please wait while we load your content...