Proof of concept for poor inhibitor binding and efficient formation of covalent adducts of KRASG12C and ARS compounds†
Abstract
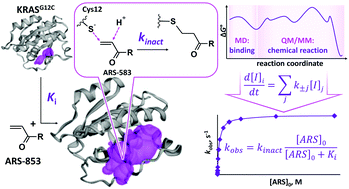
The use of selective covalent inhibitors with low binding affinity and high reactivity with the target enzyme is a promising way to solve a long-standing problem of the “undruggable” RAS-like proteins. Specifically, compounds of the ARS family that prevent the activation of the GDP-bound G12C mutant of Kirsten RAS (KRAS) are in the focus of recent experimental research. We report the first computational characterization of the entire reaction mechanism of the covalent binding of ARS-853 to the KRASG12C·GDP complex. The application of molecular dynamics, molecular docking and quantum mechanics/molecular mechanics approaches allowed us to model the inhibitor binding to the protein and the chemical reaction of ARS-853 with Cys12 in the enzyme binding site. We estimated a full set of kinetic constants and carried out numerical kinetic analysis of the process. Thus, we were able to compare directly the physicochemical parameters of the reaction obtained in silico and the macroscopic parameters observed in experimental studies. From our computational results, we explain the observed unusual dependence of the rate constant of covalent complex formation, kobs, on the ARS concentration. The latter depends both on the non-covalent binding step with the equilibrium constant, Ki, and on the rate constant of covalent adduct formation, kinact. The calculated ratio kinact/Ki = 213 M−1 s−1 reproduces the corresponding experimental value of 250 ± 30 M−1 s−1 for the interaction of ARS-853 with KRASG12C. Electron density analysis in the reactive region demonstrates that covalent bond formation occurs efficiently according to the Michael addition mechanism, which assumes the activation of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond of ARS-853 by a water molecule and Lys16 in the binding site of KRASG12C. We also refine the kinact and Ki constants of the ARS-107 compound, which shares common features with ARS-853, and show that the decrease in the kinact/Ki ratio in the case of ARS-107 is explained by changes in both Ki and kinact constants.
C bond of ARS-853 by a water molecule and Lys16 in the binding site of KRASG12C. We also refine the kinact and Ki constants of the ARS-107 compound, which shares common features with ARS-853, and show that the decrease in the kinact/Ki ratio in the case of ARS-107 is explained by changes in both Ki and kinact constants.
- This article is part of the themed collections: Chemical Biology in OBC and Mechanistic, computational & physical organic chemistry in OBC


 Please wait while we load your content...
Please wait while we load your content...