Quantification of cooperativity in the self-assembly of H-bonded rosettes†
Abstract
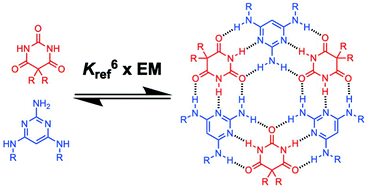
The self-assembly of triaminopyrimidines with barbiturates and with cyanates was investigated in chloroform solution. Equimolar mixtures of two complementary components form stable macrocyclic 3 : 3 complexes (rosettes). The thermodynamics of self-assembly were quantified by using 1H NMR titrations to measure the strength of pairwise H-bonding interactions between two rosette components (K), allosteric cooperativity associated with formation of a second H-bonding interaction with each component, and the effective molarity for cyclisation of the rosette motif (EM). Pyrimidine–cyanurate interactions are an order of magnitude more favourable than pyrimidine–barbiturate interactions, so the cyanurate rosettes are significantly more stable than barbiturate rosettes. There is no allosteric cooperativity associated with rosette formation, but the chelate cooperativity quantified by the product K EM is exceptionally high (102–104), indicating that there are no other species present that compete with rosette assembly. The values of EM for rosette formation are approximately 2 M for all four rosettes studied and are not affected by differences in peripheral substituents or intrinsic H-bond strength.
- This article is part of the themed collections: Celebrating our 2020 Prize and Award winners and Supramolecular chemistry in OBC


 Please wait while we load your content...
Please wait while we load your content...