The strategic use of para-quinone methides to access synthetically challenging and chemoselective α,α′-diarylmethyl N-glycosides from unprotected carbohydrate amines†
Abstract
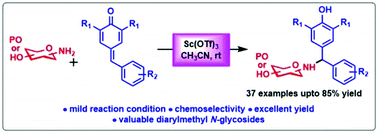
Reported herein is a practical route to access synthetically challenging and chemoselective α,α′-diarylmethyl N-glycosides via Sc(OTf)3-catalyzed 1,6-conjugate addition of amino sugars with para-quinone methides (p-QMs). The reactions proceed smoothly without a base and under mild reaction conditions with a broad substrate scope and moderate to good yields.


 Please wait while we load your content...
Please wait while we load your content...