Homoallylic amines as efficient chiral inducing frameworks in the conjugate addition of amides to α,β-unsaturated esters. An entry to enantio-enriched diversely substituted amines†
Abstract
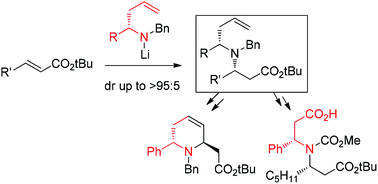
The diastereoselective conjugate addition of secondary homoallylamines, obtained in the enantioenriched form via allylmetallation of imines, to α,β-unsaturated esters is reported. This method allows access to valuable building blocks as well as heterocyclic skeletons, providing tertiary amines bearing two chains integrating a stereogenic center adjacent to the nitrogen atom.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...