Visible-light-mediated borylation of aryl and alkyl halides with a palladium complex†
Abstract
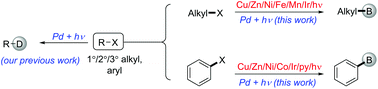
Palladium catalyzed visible-light-mediated borylation of inactivated aryl and alkyl halides is reported; the method provided high yields and excellent functional group compatibility. Furthermore, arylsilicates were synthesized selectively using dimethylphenylsilyl boronic ester via changing the reaction conditions. Finally, the possible reaction mechanism is determined through fluorescence quenching and turn on/off experiments.


 Please wait while we load your content...
Please wait while we load your content...