Access to highly enantioselective and diastereoselective spirooxindole dihydrofuran fused pyrazolones†
Abstract
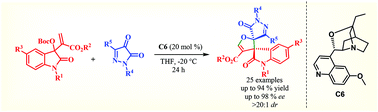
A tertiary amine catalyzed highly diastereoselective and enantioselective [3 + 2] annulation between Morita–Baylis–Hillman (MBH) carbonates derived from isatin and pyrazolone 4,5-diones has been developed. A series of structurally diverse and multifunctional spirooxindole dihydrofuran fused pyrazolone derivatives with two adjacent quaternary spirocenters has been achieved in excellent yields with good to excellent enantioselectivity. Further synthetic utility of this protocol has been successfully demonstrated by employing the bromo derivative of spirooxindole dihydrofuran fused pyrazolone to Suzuki coupling.


 Please wait while we load your content...
Please wait while we load your content...