Enantioselective synthesis of trifluoromethyl substituted cyclohexanones via an organocatalytic cascade Michael/aldol reaction†
Abstract
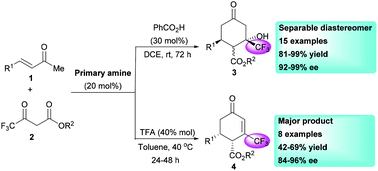
An enantioselective (92–99% ee) Michael/aldol cascade reaction between 4,4,4-trifluoroacetoacetates and α,β-unsaturated enones was established in the presence of cinchona alkaloid-based primary amines. Various β-CF3-cyclohexanones were constructed in high yields (81–99%) as a couple of separable diastereomers. This tandem reaction was sensitive to acidic co-catalysts, with a Michael/aldol condensation process favorably occurring to generate β-CF3-cyclohexenones (42–69% yield, 84–96% ee) in the presence of trifluoroacetic acid.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...