Tandem transformations and multicomponent reactions utilizing alcohols following dehydrogenation strategy
Abstract
The construction of new C–C, C–N and C–O bonds by replacing hazardous and waste generating chemicals with alcohols as the greener and sustainable reagents is one of the emerging areas of research. In consequence, the borrowing hydrogen and acceptorless dehydrogenative coupling principles have received significant momentum to synthesize various alkylated molecules and N-heterocycles. In the tandem transformations and multi-component reactions, simple substrates are directly converted to new functionalities or complex molecular systems using a single reaction set-up. In this review, the progress of tandem transformation of nitro, nitrile and azide functionalities as well as multi-component reactions utilizing alcohols is summarised. These transformations lead to the atom-economical synthesis of a wide range of alkylated imines, amines, amides and N-heterocycles such as pyrrole, pyridine, pyrimidine, quinoxaline, etc.
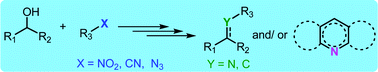
- This article is part of the themed collections: Synthetic methodology in OBC and Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...