Oxidative cyanation of 2-oxindoles: formal total synthesis of (±)-gliocladin C†
Abstract
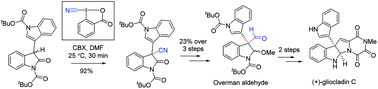
Efficient oxidative direct cyanations of 3-alkyl/aryl 2-oxindoles using Cyano-1,2-BenziodoXol-3(1H)-one (CBX) (2a) have been reported under ‘transition metal-free’ conditions to synthesize a wide variety of 3-cyano 3-alkyl/aryl 2-oxindoles sharing an all-carbon quaternary center under additive-free conditions. The application of this process is shown by the formal total synthesis of (±)-gliocladin C (11c) in a few steps.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...