Concise synthesis of thiophene C-nucleoside analogues bearing sugar residues and aromatic residues through dimerization and sulfur heterocyclization of sugar alkynes and substituted iodoethynylbenzene†
Abstract
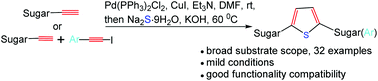
The synthesis of thiophene C-nucleoside analogues bearing sugar residues (mono- and disaccharides) and aromatic residues has been achieved by symmetric dimerization of terminal sugar alkynes or unsymmetric dimerization of terminal sugar alkynes and substituted iodoethynylbenzene followed by sulfur heterocyclization in one pot. Homocoupling of terminal sugar alkynes and subsequent sulfur heterocyclization produce thiophene C-nucleoside analogues bearing disaccharides. Unsymmetric dimerization of terminal sugar alkynes and substituted iodoethynylbenzene followed by sulfur heterocyclization give thiophene C-nucleoside analogues bearing monosaccharide and aromatic residues. This approach is concise, general and mild, and is suitable for structurally diverse pyranosides, furanosides, and acyclic sugars. Thirty-two examples have been given and the corresponding products are obtained in moderate to excellent yields.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...