Synthesis of 3-fluoro-2,5-disubstituted furans through ring expansion of gem-difluorocyclopropyl ketones†
Abstract
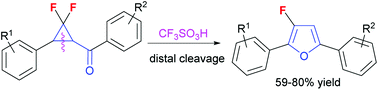
The synthesis of 3-fluoro-2,5-disubstituted furans from gem-difluorocyclopropyl ketones was accomplished using trifluoromethanesulfonic acid (CF3SO3H) through ring expansion owing to the activation of the carbonyl group in the starting material. The present synthesis of 3-fluorofurans tolerates substrates designed for products with aromatic substituents at the C-2 and C-5 positions.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...