How does cross-conjugation influence thiol additions to enones? A computational study of thiol trapping by the naturally occurring divinyl ketones zerumbone and α-santonin†
Abstract
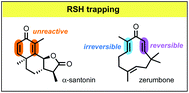
Density functional theory calculations are reported which explore how the kinetics and thermodynamics of thiol additions to enones are affected by the incorporation of the enone into a cross-conjugated divinyl ketone moiety. Computations with ωB97X-D//M06-2X indicate that in the parent acyclic system (1,4-pentadien-3-one), cross-conjugation has a small stabilizing effect on the thiol adduct, making the ΔG for the addition slightly more negative, and a larger stabilizing effect on the transition state, lowering ΔG‡. By contrast, in the parent six-membered cyclic system (2,5-cyclohexadien-1-one), cross-conjugation makes ΔG significantly less negative while causing only a small increase in ΔG‡. Both scenarios correspond to a more reversible addition. Thiol additions to two naturally occurring divinyl ketones, zerumbone and α-santonin, are examined. Previous NMR-based assays had shown that zerumbone forms mono- and bis-thiol adducts while α-santonin showed no detectable adduct formation. Computations reveal that the eleven-membered ring structure of zerumbone accelerates thiol trapping, relative to an analogous acyclic model. For α-santonin, the computations reveal that thiol addition is actually also rather facile, and it likely does occur, but the adduct is unstable and rapidly eliminates the thiol. These results illustrate that the inability to detect a thiol adduct in an experimental assay does not necessarily imply that the adduct does not form; instead it may simply be a manifestation of a rapid addition/elimination equilibrium in which the adduct concentration is below the limits of detectability.
- This article is part of the themed collection: Mechanistic, computational & physical organic chemistry in OBC


 Please wait while we load your content...
Please wait while we load your content...