1,6-Addition of vinyl p-quinone methides with cyclic sulfamidate imines: access to 4-hydroxyaryl-2,6-diarylpyridines†
Abstract
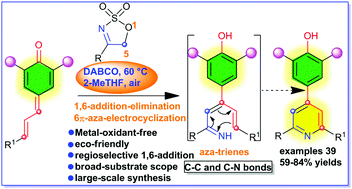
A simple and powerful one-pot regioselective 1,6-addition elimination–6π-aza-electrocyclization–aromatization reaction of vinyl/dienyl-substituted para-quinone methides with a bunch of cyclic sulfamidate imines as 2C1N synthons promoted by DABCO as a solid organobase in an open atmosphere is reported for the first time. The above-mentioned C–C and C–N bond formation process provides good to high yields of a wide range of symmetrically and unsymmetrically 2,4,6-trisubstituted pyridines possessing a sterically hindered phenolic moiety at the C4-position with a broad substrate scope. This domino [3 + 3] cyclization reaction gives rise to several compatible functionalities under metal-free conditions. Finally, the large-scale synthesis of pyridine derivatives has been demonstrated.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...