Chemical transformations of push–pull fluorenones: push–pull dibenzodicyanofulvenes as well as fluorenone– and dibenzodicyanofulvene–tetracyanobutadiene conjugates†‡
Abstract
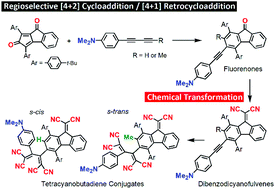
Push–pull fluorenones (FOs) were synthesized by treating a benzopentalenequinone (BPO) derivative with alkynes that bear an electron-rich aniline moiety via a regioselective [4 + 2] cycloaddition (CA) followed by a [4 + 1] retrocycloaddition (RCA). The resulting FOs were readily converted into dibenzodicyanofulvenes (DBDCFs) by treatment with malononitrile in the presence of TiCl4 and pyridine. The FOs and DBDCFs exhibit intramolecular charge-transfer (ICT) that manifests in absorptions at 350–650 nm and amphoteric electrochemical behavior. Furthermore, FOs and DBDCFs that contain a C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C bond react with tetracyanoethylene in a formal [2 + 2] CA followed by a retro-electrocyclization to afford sterically congested tetracyanobutadiene (TCBD) conjugates. The substituent (H or Me) on the aromatic ring adjacent to the butadiene moiety thereby determines whether the butadiene adopts an s-cis or s-trans conformation, and thus controls the physicochemical properties of the resulting TCBDs. The TCBD conjugates exhibit ICT absorptions (≤800 nm) together with up to four reversible reduction steps.
C bond react with tetracyanoethylene in a formal [2 + 2] CA followed by a retro-electrocyclization to afford sterically congested tetracyanobutadiene (TCBD) conjugates. The substituent (H or Me) on the aromatic ring adjacent to the butadiene moiety thereby determines whether the butadiene adopts an s-cis or s-trans conformation, and thus controls the physicochemical properties of the resulting TCBDs. The TCBD conjugates exhibit ICT absorptions (≤800 nm) together with up to four reversible reduction steps.
- This article is part of the themed collection: Mechanistic, computational & physical organic chemistry in OBC


 Please wait while we load your content...
Please wait while we load your content...